Production of 1,6-hexanediol from tetrahydropyran-2-methanol by dehydration–hydration and hydrogenation†
Abstract
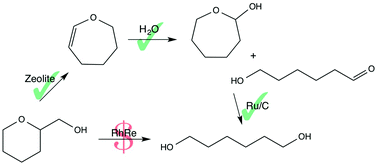
In this work we present an alternate method for the conversion of tetrahydropyran-2-methanol (THP2M), a cellulose-derived renewable building block, to 1,6-hexanediol (1,6-HDO). Our method is composed of three consecutive steps that either use relatively inexpensive catalysts or no catalyst at all. First, THP2M is catalytically dehydrated to 2,3,4,5-tetrahydrooxepine (THO) in up to 40% yield. THO is then hydrated to 2-oxepanol (OXL) and 6-hydroxyhexanal (6HDHX) with a combined yield of 85% in the absence of a catalyst. OXL and 6HDHX are then quantitatively hydrogenated to 1,6-HDO over a commercially available Ni/C or Ru/C catalyst. Various silicoaluminates were screened for the first acid-catalyzed reaction, and it was found that K-BEA shows the highest THO yield (40% over fresh catalyst, 20% after 25 h on stream). An overall 1,6-HDO yield of 34% from THP2M was obtained.


 Please wait while we load your content...
Please wait while we load your content...