Controlling reactivity through liquid assisted grinding: the curious case of mechanochemical fluorination†
Abstract
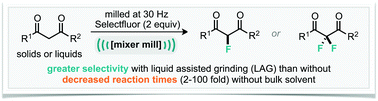
We have identified an example of a mechanochemically milled organic reaction where liquid-assisted grinding controls the selectivity, such a phenomenon has not been reported/observed before. It was found that upon milling dibenzoylmethane with Selectfluor in the absence of any solvent, a 3 : 1 ratio of monofluorinated : difluorinated product was observed. Whereas, addition of 0.125 mL of acetonitrile (∼10% of the total volume of materials present) to the ground reaction mixture afforded 50 : 1 selectivity. Furthermore, this phenomenon is applicable to a small range of diketone substrates thus far explored. Additionally, we have demonstrated that difluorination can be achieved by simply switching from adding acetonitrile to addition of sodium carbonate. Most notable, in the latter case, is the reduced reaction time compared to a conventional solvent approach, 2 hours in the mill and 24 hours in the flask.
- This article is part of the themed collection: Green Chemistry 2017 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...