Efficient synthesis of camptothecin propargylamine derivatives in water catalyzed by macroporous adsorption resin-supported gold nanoparticles†
Abstract
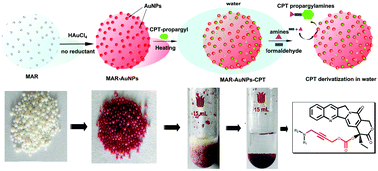
In this work, for the first time, we report an efficient, simple strategy for synthesizing active camptothecin (CPT) derivatives in water. A novel macroporous adsorption resin-supported gold nanoparticle (MAR-AuNP) was first in situ prepared in water. The resulting MAR-AuNPs swell in water and were applied not only as an efficient adsorbent for hydrophobic CPT molecules, but also as a catalyst for the Mannich reaction of CPT, formaldehyde and amines with water as a solvent. A series of novel CPT propargylamine derivatives were successfully obtained with high yields by virtue of such a strategy. Moreover, the resulting CPT propargylamine derivatives exhibited excellent cytotoxicity against two different tumor cell lines. This novel and efficient strategy, avoiding the use of toxic solvents, represents a promising green route for drug discovery from natural products and shows great potential in the green pharmaceutical industry.


 Please wait while we load your content...
Please wait while we load your content...