Pd/C catalyzed phenoxycarbonylation using N-formylsaccharin as a CO surrogate in propylene carbonate, a sustainable solvent†
Abstract
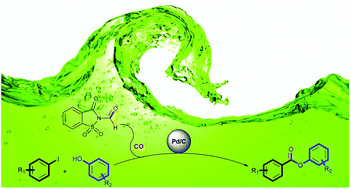
This work reports the first Pd/C catalyzed phenoxycarbonylation of aryl iodides using N-formylsaccharin as a CO surrogate. Advantageously, the reaction could be carried out in propylene carbonate, an environmentally benign and sustainable polar aprotic solvent under CO surrogacy. Using N-formylsaccharin as a CO surrogate allows the usage of cheaper and readily available phenols as the coupling partner. A range of phenyl esters could be synthesized under mild, co-catalyst free, ligand free and additive free conditions, including multi-substituted novel phenyl esters. The Pd/C catalyst could be recycled up to four times with only a slight loss in activity. The reaction could be scaled up to gram scale synthesis.


 Please wait while we load your content...
Please wait while we load your content...