Generation of wheat gluten hydrolysates with dipeptidyl peptidase IV (DPP-IV) inhibitory properties†
Abstract
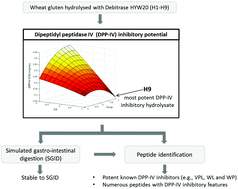
Wheat gluten, a Pro-rich dietary protein, was investigated for its potential to produce dipeptidyl peptidase IV (DPP-IV) inhibitory peptides during enzymatic hydrolysis with Debitrase HYW20. Nine gluten hydrolysates (H1–H9) were generated using a 2 factor × 3 level design of experiments (DOE) including the incubation temperature (40, 50 and 60 °C) and the enzyme: substrate ratio (E : S, 0.5, 1.0 and 1.5% (w/w)). Their DPP-IV half maximal inhibitory concentration (IC50) ranged from 0.24 ± 0.02 (H9) to 0.66 ± 0.06 mg mL−1 (H2A and H7) and their degree of hydrolysis (DH) from 31.7 ± 0.9 (H7) to 62.2 ± 3.0% (H6). Gluten and H9, the most potent DPP-IV inhibitory hydrolysate, were subjected to simulated gastrointestinal digestion (SGID), yielding Gluten_CorPP and H9_CorPP, respectively. H9_CorPP had a higher DPP-IV inhibitory potency than Gluten_CorPP (i.e., DPP-IV IC50 values of 0.33 ± 0.03 vs. 1.45 ± 0.26 mg mL−1, respectively). H9 and H9_CorPP both contained relatively potent DPP-IV inhibitory peptides such as Val-Pro-Leu, Trp-Leu and Trp-Pro which were identified by liquid chromatography tandem mass spectrometry (LC-MS/MS). In addition, several sequences possessing features of DPP-IV inhibitory peptides, mostly consisting of a penultimate or C-terminal Pro, were identified within H9. The presence of Pro-containing peptides within H9 may contribute to its stability to digestive enzymes. Gluten hydrolysates may have antidiabetic potential for humans.


 Please wait while we load your content...
Please wait while we load your content...