Metallic ion leaching from heterogeneous catalysts: an overlooked effect in the study of catalytic ozonation processes†
Abstract
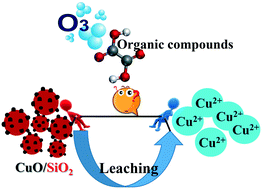
Heterogeneous catalytic ozonation has been increasingly studied for the degradation and mineralization of refractory organic water pollutants in recent years. Compared with homogeneous catalysts, an important advantage of heterogeneous catalysts is that they can be easily separated from the treated water, making the process economically viable. While many studies have focused on the development and evaluation of metal oxide-based catalytic ozonation, possible leaching of metal ions and the subsequent effect on the contaminants' degradation are sometimes overlooked. Here, we examined metal leaching from several solid catalysts and further investigated the influence of the leached metal ions on the mineralization of two model compounds (oxalate and nitrobenzene) during continuous ozonation. Metallic ion leaching was observed from both commercially-available catalysts and catalysts prepared via wet-chemistry methods in the lab. The water matrix has been demonstrated to play an important role in metal leaching. The homogeneous catalytic effect resulting from the leached metal ions was found to be significant. A mechanism involving the formation of an unstable Cu(III)/oxalate complex through the reaction between ˙OH and Cu(II)/oxalate was proposed to explain the experimental observations. Our results indicate that the stability of the solid catalysts and the effects of the leached ions must be carefully examined during the catalytic ozonation of organic contaminants. Through this study we highlight the importance of rigorous, accepted protocols for evaluating and reporting heterogeneous catalyst performance in water/wastewater treatment within the research community.


 Please wait while we load your content...
Please wait while we load your content...