Exceptional sorption behaviour of porous tungsten oxide for aqueous lead†
Abstract
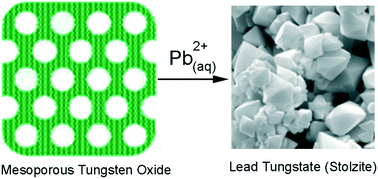
A nanoporous WO3 sorbent was found to be highly reactive towards Pb2+, exhibiting a molar sorption capacity of 42.5% and sorption half-lives as low as 47 s. The sorbent lowers the concentration of Pb2+ to less than 0.5 ppb (the instrumental detection limit), a level well below the WHO and EPA action limits. Powder X-ray diffraction and Raman spectroscopy performed on the sorbent after loading with lead demonstrated that the formation of crystalline stolzite (PbWO4) had taken place. Therefore, lead sorption occurs via a chemical reaction between Pb2+ and nanoporous WO3 accounting for the high sorption capacity. Scanning electron microscopy showed that the sorption reaction led to a large morphology change from non-descript smaller tabular particles of the sorbents to large clusters of octahedral crystallites of the stolzite product.


 Please wait while we load your content...
Please wait while we load your content...