Electro-synthesis of ammonia from nitrogen at ambient temperature and pressure in ionic liquids†
Abstract
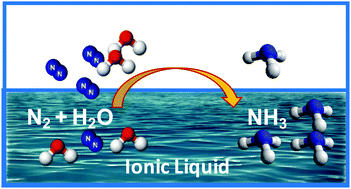
Ammonia as the source of most fertilizers has become one of the most important chemicals globally. It also is being increasingly considered as an easily transported carrier of hydrogen energy that can be generated from “stranded” renewable-energy resources. However, the traditional Haber–Bosch process for the production of ammonia from atmospheric nitrogen and fossil fuels is a high temperature and pressure process that is energy intensive, currently producing more than 1.6% of global CO2 emissions. An ambient temperature, electrochemical synthesis of ammonia is an attractive alternative approach, but has, to date, not been achieved at high efficiency. We report in this work the use of ionic liquids that have high N2 solubility as electrolytes to achieve high conversion efficiency of 60% for N2 electro-reduction to ammonia on a nanostructured iron catalyst under ambient conditions.


 Please wait while we load your content...
Please wait while we load your content...