A general polymer-assisted strategy enables unexpected efficient metal-free oxygen-evolution catalysis on pure carbon nanotubes†
Abstract
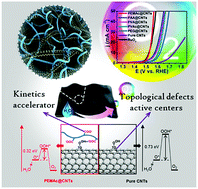
A conceptually new and general strategy was, for the first time, proposed to significantly boost the electrocatalytic activity of metal-free pure carbon nanotubes (CNTs) towards the oxygen evolution reaction (OER) by simple polymer wrapping without introducing any heteroatom dopants, functional groups, or edge defects into the graphitic structure. Our strategy is straightforward, efficient, green, and easy to be scaled up. After wrapping pure CNTs with a certain class of electrochemically inert polymers (i.e. poly(ethylene-alt-maleic acid), poly(vinyl alcohol), poly(vinyl acetate), poly(ethylene glycol)) with polar oxygen-containing groups (i.e. –COOH, –OH, –COOCH3, –O–) through noncovalent interactions, a series of advanced metal-free composite membrane catalysts were easily achieved, which yielded unexpected, surprisingly high OER activity – on par with the commercial noble RuO2 catalyst, though pure CNTs have rather poor OER activity. Combined experimental and computational studies revealed that the observed superb OER activity could be attributed to a synergistic effect of intrinsic topological defects in the CNTs as active centers and the coated polymer layer as a co-catalyst to optimize the adsorption energies of intermediates for improving the OER energetics.
- This article is part of the themed collection: 2017 Energy and Environmental Science HOT articles


 Please wait while we load your content...
Please wait while we load your content...