Crystalline nickel manganese antimonate as a stable water-oxidation catalyst in aqueous 1.0 M H2SO4†
Abstract
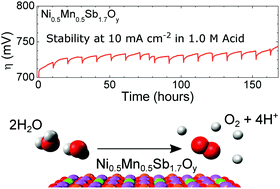
Water oxidation is a required half-reaction for electrochemical water splitting. To date, the only well-established active oxygen-evolution catalysts stable under operating conditions and at rest in acidic aqueous media contain Ru or Ir, two of the scarcest non-radioactive elements on Earth. We report herein a nickel-manganese antimonate electrocatalyst with a rutile-type crystal structure that requires an initial voltammetric overpotential of 672 ± 9 mV to catalyze the oxidation of water to O2(g) at a rate corresponding to 10 mA cm−2 of current density when operated in contact with 1.0 M sulfuric acid. Under galvanostatic control, the overpotential initially rose from 670 mV but was then stable at 735 ± 10 mV for 168 h of continuous operation at 10 mA cm−2. We additionally provide an in-depth evaluation of the stability of the nickel-manganese antimonate electrocatalyst, including elemental characterization of the surface, bulk, and electrolyte before and after electrochemical operation.


 Please wait while we load your content...
Please wait while we load your content...