NO˙ disproportionation by a {RhNO}9 pincer-type complex†
Abstract
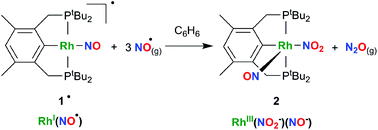
The reactivity of the {RhNO}9 complex [Rh(PCPtBu)(NO)]˙ (1˙) with NO˙ was studied. A disproportionation reaction takes place in which N2O is released quantitatively, while the complex Rh(PCPtBu)(NO)(NO2) (2), with coordinated nitrite, is formed. The new complex 2 was fully characterized by multinuclear NMR techniques, IR and X-ray diffraction. The X-ray structure reveals a square pyramidal geometry with an N-bound nitro ligand trans to the Cipso of the PCP ligand and a bent nitrosyl ligand in the apical position. IR measurement of released N2O confirms that one equivalent forms for each molecule of 1˙. Infrared spectroscopic experiments with 1˙-15NO and 14NO˙ suggest that the reaction occurs through the intermediacy of a dinitrosyl complex. In addition, DFT calculations were performed to provide more evidence on the structure of the intermediates and to support the observed reactivity.


 Please wait while we load your content...
Please wait while we load your content...