Redox non-innocent bis(2,6-diimine-pyridine) ligand–iron complexes as anolytes for flow battery applications†
Abstract
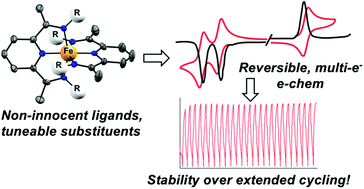
Diiminepyridines are a well-known class of “non-innocent” ligands that confer additional redox activity to coordination complexes beyond metal-centred oxidation/reduction. Here, we demonstrate that metal coordination complexes (MCCs) of diiminepyridine (DIP) ligands with iron are suitable anolytes for redox-flow battery applications, with enhanced capacitance and stability compared with bipyridine analogs, and access to storage of up to 1.6 electron equivalents. Substitution of the ligand is shown to be a key factor in the cycling stability and performance of MCCs based on DIP ligands, opening the door to further optimization.


 Please wait while we load your content...
Please wait while we load your content...