Ferromagnetic coupling in copper benzimidazole chloride: structural, mass spectrometry, magnetism, and DFT studies†
Abstract
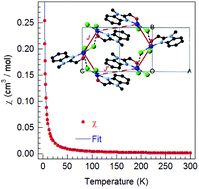
Herein, quasi-square planar CuII(Hmbm)Cl2 (CBC, Hmbm = (1-methyl-1H-benzo[d]imidazol-2-yl)methanol) was arranged in a pseudo orthogonal way to form Cl-bridged chains, and further π⋯π interactions resulted in distorted hexagonal layers. DFT calculations reveal a bond strength order of Cu–Cl > Cu–O/N ≫ Cu⋯Cl. ESI-MS data reveal several small fragments from CBC, but oligomeric [CuII2], [CuII3], and [CuII4] for non-zero in-source energies; MS data indicates the occurrence of several chemical processes, viz. splitting of the ligand, oligomerization, and redox reaction of alcohol to aldehyde and CuII to CuI. Gibbs free energies for the fragments were estimated using DFT. The magnetic susceptibility was modeled with the ferromagnetic coupling J(Cu–Cl2a⋯Cu) = +0.99(30) cm−1 and J′(π⋯π) = +0.35(16) cm−1 and g = 2.38(2). HF-EPR determined the anisotropic g-values, gx = 2.24, gy = 2.16, and gz = 2.09, and a hyperfine constant of Az = 450 G. DFT calculations from crystal structure data reveal a J(Cu–Cl2a⋯Cu) of +3.6 at 296 K and +4.1 cm−1 at 90 K that dominates the magnetic properties, whereas J′(π⋯π) = 0.04 cm−1 is negligibly small.


 Please wait while we load your content...
Please wait while we load your content...