Separation of Am3+ and Eu3+ using hexa-n-octylnitrilo triacetamide (HONTA): complexation, extraction, luminescence, EXAFS and DFT studies†
Abstract
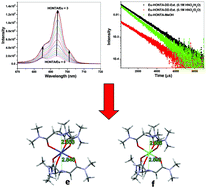
This paper reports the solvent extraction of Am3+ and Eu3+ using N,N,N′,N′,N′′,N′′-hexa-n-octylnitrilotriacetamide (HONTA) as the extractant in n-dodecane. The results are in variance with those reported previously with respect to the nature of the extracted species. The solvent extraction data were entirely different from those reported previously as the extracted species conformed to 1 : 2 (M : L) species for both Am3+ and Eu3+ ions. The structure of the extracted complex was determined by EXAFS demonstrating the three amidic ‘O’ atoms of the HONTA complex with the Eu3+ ion. In the case of the Am3+ ion, the pivotal ‘N’ atom is suggested to bond to the metal ion, which may explain the significantly more favourable extraction of Am3+vis-à-vis Eu3+. The absence of H2O molecules in the inner coordination sphere of the Eu3+–HONTA extract was confirmed by luminescence spectroscopic measurements. Complexation studies in MeOH and EtOH indicated the formation of both 1 : 1 and 1 : 2 complexes with Nd3+ ions. The results are explained on the basis of DFT calculations using HMNTA, the corresponding hexamethyl analogue of HONTA.


 Please wait while we load your content...
Please wait while we load your content...