Novel core–shell-like nanocomposites xCu@Cu2O/MgAlO-rGO through an in situ self-reduction strategy for highly efficient reduction of 4-nitrophenol†
Abstract
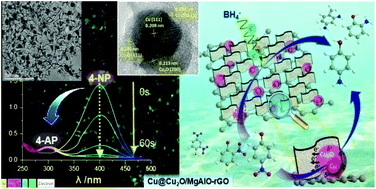
A series of novel hierarchical nanocomposite catalysts xCu@Cu2O/MgAlO-rGO were fabricated by calcination of CuxMg3−xAl-LDH/rGO precursors (LDH: layered double hydroxide, rGO: reduced graphene oxide, and x = 0.5, 1.0, and 1.5), obtained by a facile citric acid-assisted coprecipitation route, under a N2 flow upon in situ self-reduction of lattice atomic-dispersed Cu2+ by rGO. Systematic characterization reveals highly dispersed core–shell-like Cu@Cu2O nanoparticles near the border between vertically interconnected mixed oxide MgAlO nanoplates and rGO layers. All the obtained catalysts show extraordinary catalytic performances for the reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) at room temperature. The 1.0Cu@Cu2O/MgAlO-rGO shows the highest activity for complete conversion of 4-NP with an apparent rate constant (kapp) of 55.3 × 10−3 s−1, a normalized rate constant (knor) of 14 497 s−1 g−1 on an active Cu content, and an unprecedented recycling stability for 25 successive cycles, which are superior to those of the recently reported Cu- and Co-based metal nanoparticles and even compared favourably with those of the most active noble metal catalysts. The superior activity of 1.0Cu@Cu2O/MgAlO-rGO can be attributed to the highly dispersed core–shell-like Cu@Cu2O nanoparticles and the greatly enhanced four-phase synergistic effect among Cu, Cu2O, MgAlO and rGO upon calcination. Moreover, 1.0Cu@Cu2O/MgAlO-rGO shows an excellent efficiency in the fixed bed system for the treatment of simulated industrial effluents containing nitrophenols and organic dyes. The present cost-effective, highly efficient and reusable non-noble metal nanocatalyst would open a new pathway for future water remediation.


 Please wait while we load your content...
Please wait while we load your content...