A carbon-free inorganic–metal complex consisting of an all-nitrogen pentazole anion, a Zn(ii) cation and H2O†
Abstract
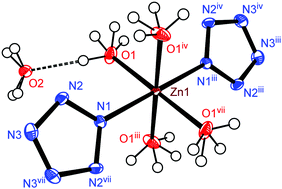
A carbon-free inorganic–metal complex [Zn(H2O)4(N5)2]·4H2O was synthesized by the ion metathesis of [Na(H2O)(N5)]·2H2O solution with Zn(NO3)2·6H2O. The complex was well characterized by IR and Raman spectroscopy, elemental analysis (EA), powder X-ray diffraction (PXRD), and differential scanning calorimetry (DSC). The structure of the complex was confirmed by single-crystal X-ray crystallography and a Zn(II) ion is coordinated in a quadrilateral bipyramid environment in which the axial position is formed by two nitrogen atoms (N1) from two pentazolate rings (cyclo-N5−) and the equatorial plane is formed by four oxygen atoms (O1) from four coordinated water molecules. The thermal analysis of [Zn(H2O)4(N5)2]·4H2O reveals that although water plays an important role in stabilizing cyclo-N5−, dehydration does not cause immediate decomposition of the anion. However, cyclo-N5− decomposed into N3− and N2 gas at 107.9 °C (onset). Based on its chemical compatibility and stability, the complex exhibits promising potential as a modern environmentally-friendly energetic material.


 Please wait while we load your content...
Please wait while we load your content...