One-pot synthesis of imidazolinium salts via the ring opening of tetrahydrofuran†
Abstract
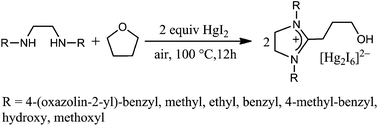
A new one-pot synthesis of C2-hydroxypropyl-substituted imidazolinium salts via the ring opening of tetrahydrofuran (THF) with N,N′-disubstituted diamines has been developed. Preliminary studies of the reaction mechanism suggest the CO2-promoted oxidative ring opening of THF followed by Hg(II)-mediated oxidation of an imidazolidine intermediate. These novel C2-substituted imidazolinium salts have shown to be active catalysts for the aza-Diels–Alder reactions.


 Please wait while we load your content...
Please wait while we load your content...