Strontium-coordination polymers based on tetrafluorophthalic and phthalic acids: mechanochemical synthesis, ab initio structures determination, and spectroscopic characterization†
Abstract
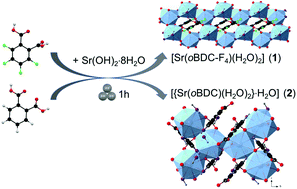
Two strontium-based dicarboxylate systems [Sr(oBDC-F4)(H2O)2] (1) and [{Sr(oBDC)(H2O)2}·H2O] (2) were synthesized mechanochemically via milling of Sr(OH)2·8H2O with tetrafluorophthalic acid (H2oBDC-F4) or phthalic acid (H2oBDC), respectively. The new structures were determined ab initio from the powder X-ray diffraction (PXRD) data. Both compounds 1 and 2 crystallize in the monoclinic space group P21/c as two-dimensional coordination polymers (2D-CPs). The determined structures were validated by extended X-ray absorption (EXAFS) data. Compounds 1 and 2 show different thermal stabilities. The fluorinated CP 1 is decomposed at 300 °C while the nonfluorinated CP 2 transforms into a new phase after thermal treatment at 400 °C. The two hydrated CPs exhibit small surface areas which increase after the thermal post-treatment for 1 but remains unchanged for the dehydrated sample of 2. Dynamic vapor sorption (DVS) experiments indicate that both the dehydrated and hydrated samples of 2 depict no significant differences in their adsorption isotherms. The DVS of water indicates that the phase transition after thermal post-treatment of 2 is irreversible.


 Please wait while we load your content...
Please wait while we load your content...