Perovskite alkali metal samarium borohydrides: crystal structures and thermal decomposition†
Abstract
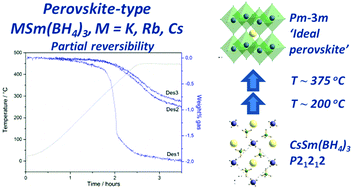
A new synthesis method of samarium borohydride, Sm(BH4)2, using tetrahydrofuran borane, THF-BH3, and samarium hydride, SmH2, has been demonstrated and verified. The synthesised Sm(BH4)2 was mechanochemically treated with MBH4, M = K, Rb, Cs. Initially, the formation of KSm(BH4)3 is observed while subsequent heat treatment is necessary to form MSm(BH4)3, M = Rb, Cs. The new compounds crystallise in orthorhombic unit cells adopting perovskite-type 3D frameworks containing distorted [Sm(BH4)6] octahedra. In situ X-ray diffraction studies reveal two second-order polymorphic transitions of α-CsSm(BH4)3via a tetragonal intermediate, α′-CsSm(BH4)3, into a cubic high-temperature polymorph, β-CsSm(BH4)3, resembling an ideal perovskite structure. The new compounds, MSm(BH4)3, are thermally stable up to T ∼ 280 °C after which they decompose into mainly MBH4, SmH2 and possibly SmB6 and SmB12H12. Finally, after three cycles of hydrogen release and uptake, the storage capacity was 1.0 wt% for KSm(BH4)3 and 0.84 wt% for RbSm(BH4)3 and CsSm(BH4)3.


 Please wait while we load your content...
Please wait while we load your content...