Aqueous chemistry of Ce(iv): estimations using actinide analogues†
Abstract
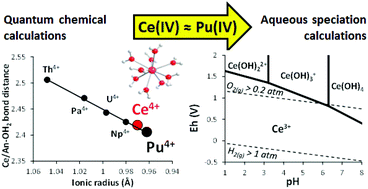
The prediction of cerium (Ce) aqueous speciation is relevant in many research fields. Indeed, Ce compounds are used for many industrial applications, which may require the control of Ce aqueous chemistry for their synthesis. The aquatic geochemistry of Ce is also of interest. Due to its growing industrial use and its release into the environment, Ce is now considered as an emerging contaminant. Cerium is also used as a proxy of (paleo)redox conditions due to the Ce(IV)/Ce(III) redox transition. Finally, Ce(IV) is often presented as a relevant analogue of tetravalent actinides (An(IV)). In the present study, quantum chemical calculations were conducted to highlight the similarities between the structures of Ce(IV) and tetravalent actinide (An(IV); An = Th, Pa, U, Np, Pu) aqua-ions, especially Pu(IV). The current knowledge of An(IV) hydrolysis, solubility and colloid formation in water was briefly reviewed but important discrepancies were observed in the available data for Ce(IV). Therefore, new estimations of the hydrolysis constants of Ce(IV) and the solubility of Ce(IV)-(hydr)oxides are proposed, by analogy with Pu(IV). By plotting pH–Eh (Pourbaix) diagrams, we showed that the pH values corresponding to the onset of Ce(IV) species formation (i.e. Ce(IV)-(hydr)oxide or dissolved Ce(IV)) agreed with various experimental results. Although further experimental studies are required to obtain a more accurate thermodynamic database, the present work might yet help to predict more accurately the Ce chemical behavior in aqueous solution.


 Please wait while we load your content...
Please wait while we load your content...