Structural variations in (CuL)2Ln complexes of a series of lanthanide ions with a salen-type unsymmetrical Schiff base(H2L): Dy and Tb derivatives as potential single-molecule magnets†
Abstract
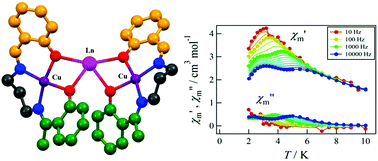
A new series of heterometallic trinuclear Cu2Ln complexes [lanthanide ions Ln = Gd (1), Tb (2), Dy (3), Ho (4) and Er (5)] has been synthesized using a Cu(II)-metalloligand derived from a N2O2 donor unsymmetrical Schiff base, H2L (where H2L = N-α-methylsalicylidene-N′-salicylidene-1,3-propanediamine), and structurally characterized. Among these complexes, [(CuL)2Gd(NO3)3(CH3CN)2] (1), [(CuL)2Tb(NO3)3(CH3CN)2] (2) and [(CuL)2Dy(NO3)3(CH3CN)2] (3) are isomorphic and isostructural. In these complexes two metalloligands coordinate to the central Ln(III) (Ln = Gd, Tb and Dy respectively) ion in a transoid fashion via μ2-phenoxido oxygen atoms. The Ln(III) ions are deca-coordinated with a distorted tetradecahedron geometry. The two terminal Cu(II) ions of the complexes possess a hexa-coordinated distorted octahedral geometry. In contrast, in complexes [(CuL)2Ho(NO3)3(CH3CN)], (4) and [(CuL)2Er(NO3)3(CH3CN)]·0.5(CH3CN) (5), the two metalloligands coordinated to the Ln(III) ions in a cisoid fashion. The Ho(III) ion in 4 is nona-coordinated with a distorted tricapped trigonal prismatic geometry and the Er(III) ion in 5 is octa-coordinated with a distorted square antiprismatic geometry. The two terminal Cu(II) ions in complexes 4 and 5 are penta-coordinated with a distorted square-pyramidal geometry. The dc magnetic susceptibilities and field dependent magnetization measurement of complex 1 reveal the occurrence of ferromagnetic interactions between Cu(II) and Gd(III) ions as well as intermolecular antiferromagnetic interactions. Both complexes 2 and 3 show ferromagnetic interactions between Cu(II) and Ln(III) ions. The ac magnetic susceptibilities of all the complexes were also recorded and it was found that only complexes 2 and 3 exhibit slow relaxation of magnetization reorientation below 10 K at 2000 Oe applied dc field, this being characteristic of single molecule magnets.

 Please wait while we load your content...
Please wait while we load your content...