Copper complexes with dissymmetrically substituted bis(thiosemicarbazone) ligands as a basis for PET radiopharmaceuticals: control of redox potential and lipophilicity†
Abstract
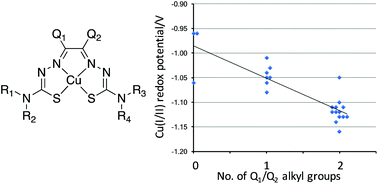
Copper(II) bis(thiosemicarbazone) derivatives have been used extensively in positron emission tomography (PET) to image hypoxia and blood flow and to radiolabel cells for cell tracking. These applications depend on control of redox potentials and lipophilicity of the bis(thiosemicarbazone) complexes, which can be adjusted by altering peripheral ligand substituents. This paper reports the synthesis of a library of new dissymmetrically substituted bis(thiosemicarbazone) ligands by controlling the condensation reactions between dicarbonyl compounds and 4-substituted-3-thiosemicarbazides or using acetal protection. Copper complexes of the new ligands have been prepared by reaction with copper acetate or via transmetallation of the corresponding zinc complexes, which are convenient precursors for the rapid synthesis of radio-copper complexes. Well-defined structure–activity relationships linking ligand alkylation patterns with redox potential and lipophilicity of the complexes are reported.
- This article is part of the themed collection: Frontiers in Radionuclide Imaging and Therapy


 Please wait while we load your content...
Please wait while we load your content...