A Schiff base platform: structures, sensing of Zn(ii) and PPi in aqueous medium and anticancer activity†
Abstract
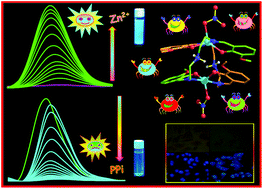
A reaction of N1,N3-bis(3-methoxysalicylidene) diethylenetriamine (H2Vd) and Zn(NO3)2·6H2O, ZnBr2, ZnI2 and Cd(NO3)2·4H2O in a methanol solution led to zinc and cadmium complexes of different nuclearities, [Zn2(Vd·H)2(X)2]·CH3OH (X = NO3, Br, I) [1a, 1b and 1c] and Cd3(Vd)2(NO3)2 (2). In 1(a–c), two H2Vd ligands bridge the two metal centers whereas in 2, they provide sideways support to two terminal Cd2+ ions, providing an all-oxygen envelope to the central Cd2+ ion. All four compounds were characterized by elemental analysis, FT-IR spectroscopy and single crystal X-ray diffraction analysis. Complexes 1(a–c) exhibit dinuclear structures, whereas 2 exhibits a nearly linear trinuclear structure. The structural differences among these complexes are attributable to various coordination modes and flexible configurations of the H2Vd ligand. The ligand H2Vd is an excellent probe for sensing Zn2+ in solution, whereas complexes 1(a–c) are able to selectively detect pyrophosphate (PPi) in aqueous medium. The structure of the pyrophosphate (PPi) complex has been proposed using DFT calculations and the selectivity is due to the unique ability of this anion to simultaneously coordinate to both the Zn metal centers. The anticancer activity of complexes 1(a–c) was also explored.


 Please wait while we load your content...
Please wait while we load your content...