Heteroleptic nickel(ii)–diNHC complexes and an unusual ‘reverse’ carbene-transfer reaction to silver(i) †
Abstract
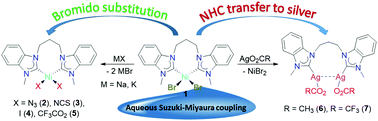
A series of rare [NiX2(MeCCprop)] complexes bearing the cis-chelating benzimidazole-derived dicarbene ligand MeCCprop and varying anionic coligands (2, X = N3; 3, X = NCS; 4, X = I; 5, X = O2CCF3) have been prepared and coligand dependent structural and spectroscopic features have been evaluated. This study also revealed an unusual ‘reverse’ carbene transfer reaction from nickel to silver giving the disilver species [Ag2X2(μ–κ2-MeCCprop)] (6, X = OAc; 7, X = O2CCF3). A preliminary catalytic study of two representative NiII diNHC complexes in the aqueous and phosphine-free Suzuki–Miyaura coupling reaction of aryl halides is reported as well. These reactions provide good yields of coupling products, but do not require inert conditions.


 Please wait while we load your content...
Please wait while we load your content...