Substitution reactions of iron(ii) carbamoyl-thioether complexes related to mono-iron hydrogenase†
Abstract
A C,N,S pincer complex has been synthesized for structural modeling of the organometallic active site of mono-[Fe] hydrogenase (HMD). The C,N,S chelate allows for systematic investigation of the substitution reactions of CO and other exogenous X/L-type ligands, as well as examination of the exact roles of the Fe–carbamoyl and {Fe(CO)2}2+ units in stabilizing the low-spin Fe(II) center. Reaction of the ‘apo-ligand’ 6-(2-(methylthio)phenyl)pyridin-2-amine (H2NNpySMe) with [Fe(CO)4(Br)2] affords the organometallic complex [(O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNHNpySMe)Fe(CO)2(Br)] (1). Facile substitutions of the halide with L-type ligands such as MeCN, PR3 (R = C6H5, OEt, Me), pyridine and tBuNC afford diamagnetic cations of the type [(O
CNHNpySMe)Fe(CO)2(Br)] (1). Facile substitutions of the halide with L-type ligands such as MeCN, PR3 (R = C6H5, OEt, Me), pyridine and tBuNC afford diamagnetic cations of the type [(O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNHNpySMe)Fe(CO)2(L)]+ (2a–f). Treatment of 1 with Na[S(2,6-Me2C6H3)] affords the neutral complex [(O
CNHNpySMe)Fe(CO)2(L)]+ (2a–f). Treatment of 1 with Na[S(2,6-Me2C6H3)] affords the neutral complex [(O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNHNpySMe)Fe(CO)2(S(2,6-Me2C6H3))] (2g). Substitution for CO ligand(s) was achieved with trimethylamine-N-oxide (TMAO), and in the presence of PPh3 or pyridine it afforded the six-coordinate monocarbonyl complexes [(O
CNHNpySMe)Fe(CO)2(S(2,6-Me2C6H3))] (2g). Substitution for CO ligand(s) was achieved with trimethylamine-N-oxide (TMAO), and in the presence of PPh3 or pyridine it afforded the six-coordinate monocarbonyl complexes [(O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNHNpySMe)Fe(CO)(Br)(PPh3)] (3a), [(O
CNHNpySMe)Fe(CO)(Br)(PPh3)] (3a), [(O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNHNpySMe)Fe(CO)(PPh3)2](BArF4) (3b), and [(O
CNHNpySMe)Fe(CO)(PPh3)2](BArF4) (3b), and [(O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNHNpySMe)Fe(CO)(py)2](BArF4) (3c). Interestingly the stable low-spin Fe(II), 5-coordinate complex of the formula [(O
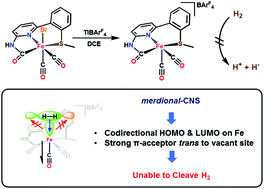
CNHNpySMe)Fe(CO)(py)2](BArF4) (3c). Interestingly the stable low-spin Fe(II), 5-coordinate complex of the formula [(O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNHNpySMe)Fe(CO)2](BArF4) (4) was accessed by treating 1 with TlBArF4 in non-coordinating solvents (DCE, FPh); notably, 4 does not react with H2 in the presence (or absence) of a base. To elucidate the electronic structure differences between the five-coordinate versus six-coordinate complexes, DFT calculations for 4 and 1 were performed. Geometry optimization indicates that 4+ maintains a square-pyramidal geometry, and the Hessian calculation accurately simulates the ν(C
CNHNpySMe)Fe(CO)2](BArF4) (4) was accessed by treating 1 with TlBArF4 in non-coordinating solvents (DCE, FPh); notably, 4 does not react with H2 in the presence (or absence) of a base. To elucidate the electronic structure differences between the five-coordinate versus six-coordinate complexes, DFT calculations for 4 and 1 were performed. Geometry optimization indicates that 4+ maintains a square-pyramidal geometry, and the Hessian calculation accurately simulates the ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O) in 4+. The electronic structure of 4+ predicts that the HOMO (comprised of Fe|Ncarb) and LUMO (Fe only) orbitals in 4+ are properly oriented to interact with an incoming ligand. However, we postulate that codirectional orientation of the HOMO and LUMO orbitals explains the lack of H2 reactivity with this equatorial CNS donor set, despite many other structural similarities to the endogenous active site. Based on a related work from our lab, we conclude that a facial C,N,S coordination mode is necessary to promote H2 activation and cleavage.
O) in 4+. The electronic structure of 4+ predicts that the HOMO (comprised of Fe|Ncarb) and LUMO (Fe only) orbitals in 4+ are properly oriented to interact with an incoming ligand. However, we postulate that codirectional orientation of the HOMO and LUMO orbitals explains the lack of H2 reactivity with this equatorial CNS donor set, despite many other structural similarities to the endogenous active site. Based on a related work from our lab, we conclude that a facial C,N,S coordination mode is necessary to promote H2 activation and cleavage.


 Please wait while we load your content...
Please wait while we load your content...