Influence of intramolecular vs. intermolecular phosphonium-borohydrides in catalytic hydrogen, hydride, and proton transfer reactions†
Abstract
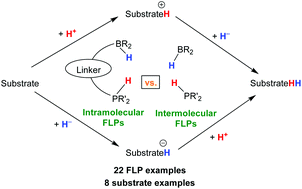
In this computational study, the thermodynamics of hydrogen, hydride, and proton transfer from 22 phosphonium-borohydride intramolecular and intermolecular frustrated Lewis pairs (FLPs) to eight probe substrates was investigated. The purpose of this study was to gain insight into the thermodynamics of H2 transfer with intramolecular phosphonium-borohydrides; to determine whether intramolecular or intermolecular FLPs are preferred in FLP-catalyzed hydrogenation reactions. Comparison of the computed thermodynamic values showed that by connecting a borohydride and phosphonium center through a linker, H2 loss from the respective intramolecular phosphonium-borohydride became less favorable by about five and seven kcal mol−1 in acetonitrile and toluene, respectively. Connecting the borohydride and phosphonium centers also resulted in both hydride and proton loss becoming less favorable, on average, by about 10.0 kcal mol−1 and about 4.6 pKa units, respectively. Analysis of hydrogen, proton, and hydride transfer to eight probe substrates showed that initial proton transfer is 49 and 20 kcal mol−1 more favorable than the initial hydride transfer in the reduction of nitrogen-containing and oxygen-containing unsaturated substrates, respectively. These results suggest that proton transfer, followed by hydride transfer occurs in the reduction of imines, ketones, aldehydes, and enamines. From the thermodynamic analysis of proton and hydride transfer, an intramolecular phosphonium-borohydride was the desired catalyst for the reduction of imines and enamines, while an intermolecular phosphonium-borohydride was the favored catalyst for the reduction of ketones and aldehydes.


 Please wait while we load your content...
Please wait while we load your content...