Rhenium-catalysed hydroboration of aldehydes and aldimines†
Abstract
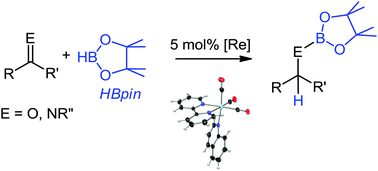
The first examples for the rhenium-catalysed hydroboration of aldehydes, ketones and aldimines, including heteroaromatic quinoline, are reported herein. Reactions are remarkably chemoselective and tolerant of several functional groups. A wide array of rhenium complexes were efficient pre-catalysts for these hydroborations, including new low-valent complexes of the formula [Re(N–N)(CO)3(L)]X (N–N = bipy derivative, L = labile ligand/solvent, and X = [BArF4]− and [B(3,5-di-tBu-cat)2]−), which have been characterized fully including an X-ray diffraction study for [Re(bipy)(CO)3(quin)][BArF4] (2). A new silver spiroboronate ester Ag[B(3,5-di-tBu-cat)2](NCCH3)3 (3) was prepared and characterized fully, including an X-ray diffraction study, and used to make one of the new rhenium complexes.


 Please wait while we load your content...
Please wait while we load your content...