Phosphomolybdate assembly as a low-cost catalyst for the reduction of toxic Cr(vi) in aqueous solution†
Abstract
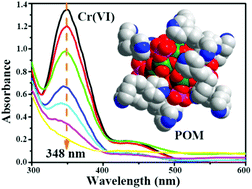
Highly reduced phosphomolybdate hybrid compounds have been hydrothermally prepared and structurally defined. X-ray single-crystal analysis revealed that these phosphomolybdates had the following formulae: (H2bpp)6{Fe[Mo6O12(OH)3(HPO4)2(H2PO4)2]2}2·11H2O (1), (H2bpp)6{Zn[Mo6O12(OH)3(HPO4)2(H2PO4)2]2}2·11H2O (2), and (H2bpp)5.5{Mn[Mo6O12(OH)3(HPO4)2(H2PO4)2]2}2·2H2O (3) (bpp = 1,3-bi(4-pyridyl)propane). In the inorganic moiety, all Mo centers are in the +5 reduced form, representing a unique, fully reduced cluster in the polyanionic family. As a counter cation, the flexible bpp surrounds the anionic cluster to form hybrid ‘core–shell’ supramolecular assemblies. These hybrids are stable and insoluble in water, which can change a catalytic reaction from homogeneous to heterogeneous. Experiments showed that the three supramolecular hybrids exhibit ideally reversible multi-electron transfer behavior, and hybrid 1 is active as a heterogeneous molecular catalyst to reduce toxic Cr(VI) to nontoxic Cr(III) at low temperature in a short period of time. Hybrid 1 serves as an electron shuttle to promote the redox reaction between Cr(VI) and HCOOH. The activation energy of the reaction is effectively decreased after the reactants are adsorbed on the solid crystal surface, and the activation energy is calculated to be 78.5 kJ·mol−1. The well-organized structures of polyoxometalates help to explain the catalytic mechanism at the molecular level.


 Please wait while we load your content...
Please wait while we load your content...