Disilametallacyclic chemistry for efficient catalysis
Abstract
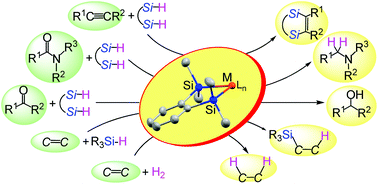
This article discusses two new features of disilametallacyclic chemistry that contribute to the development of efficient catalytic reactions in organic synthesis. The first is disilametallacyclic intermediates in the hydrosilane reduction of carbonyl compounds. Experimental and theoretical studies on disilaplatinacycles suggested that the H2Pt(IV)Si2 species generated by oxidative addition of 1,2-bis(dimethylsilyl)benzene behaves as a highly reactive hydride to reduce amides to amines. This mechanism via disilametallacyclic intermediates explains the efficient hydrosilane reduction of carbonyl compounds with α,ω-bifunctional hydrosilanes catalyzed by other transition metals. The second is hydrogenation of alkenes by disilaferra- or disilaruthenacyclic complexes as catalyst precursors. A new mechanism not involving the conventional oxidative addition of H2 was suggested from DFT calculations, in which activation of the H–H bond occurs in the metal–silicon bond of the disilametallacyclic intermediate. Disilametallacyclic intermediates contribute to efficient catalytic reactions through this σ-CAM (σ-complex assisted mechanism) type mechanism.
- This article is part of the themed collection: 2017 Frontier and Perspective articles


 Please wait while we load your content...
Please wait while we load your content...