From polyethylene waxes to HDPE using an α,α′-bis(arylimino)-2,3:5,6-bis(pentamethylene)pyridyl-chromium(iii) chloride pre-catalyst in ethylene polymerisation†
Abstract
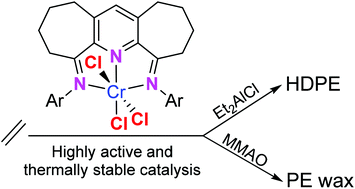
Five examples of α,α′-bis(arylimino)-2,3:5,6-bis(pentamethylene)pyridyl-chromium(III) chlorides (aryl = 2,6-Me2Ph Cr1, 2,6-Et2Ph Cr2, 2,6-i-Pr2Ph Cr3, 2,4,6-Me3Ph Cr4, 2,6-Et2-4-MePh Cr5) have been synthesized by the one-pot template reaction of α,α′-dioxo-2,3:5,6-bis(pentamethylene)pyridine, CrCl3·6H2O and the corresponding aniline. The molecular structures of Cr1 and Cr4 reveal distorted octahedral geometries with the N,N,N-ligand adopting a mer-configuration. On activation with an aluminium alkyl co-catalyst, Cr1–Cr5 exhibited high catalytic activities in ethylene polymerization and showed outstanding thermal stability operating effectively at 80 °C with activities up to 1.49 × 107 g of PE (mol of Cr)−1 h−1. Significantly, the nature of the co-catalyst employed had a dramatic effect on the molecular weight of the polymeric material obtained. For example, using diethylaluminium chloride (Et2AlCl) in combination with Cr4 gave high density/high molecular weight polyethylene with broad molecular weight distributions (30.9–39.3). By contrast, using modified methylaluminoxane (MMAO), strictly linear polyethylene waxes of lower molecular weight and narrow molecular weight distribution (1.6–2.0) were obtained with vinyl end-groups.


 Please wait while we load your content...
Please wait while we load your content...