Potassium complexes supported by monoanionic tetradentate amino-phenolate ligands: synthesis, structure and catalysis in the ring-opening polymerization of rac-lactide†
Abstract
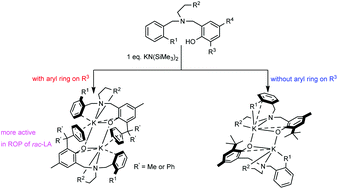
A series of potassium complexes bearing monoanionic tetradentate amino-phenolate ligands, [LK]2 (L = {(2-R1)C6H4CH2N[(CH2)2R2]CH2(4-R4-6-R3)C6H2O-}, R1 = NMe2, R2 = NEt2, R3 = CPh3, R4 = Me (1); R1 = R2 = NEt2, R3 = CPh3, R4 = Me (2); R1 = NMe2, R2 = NEt2, R3 = R4 = cumyl (4); R1 = R2 = OMe, R3 = tBu, R4 = Me (6); L = (2-NMe2)C6H4CH2N[[CH2-(S)-1-butylpyrrolidinyl]CH2(4-Me-6-CPh3)C6H2O-] (3)), have been synthesized via reactions of KN(SiMe3)2 and 1 equiv. of the corresponding aminophenols. The solid-state structures of typical complexes 4 and 6 are determined via X-ray diffraction studies, which reveal the dinuclear nature of these complexes. By contrast, DOSY measurements of 1, 4 and 6 suggest that these complexes are monomeric in solution. It is noteworthy that the coordination chemistry of these potassium complexes is versatile, which is closely related to the nature of the ortho-substituent of the phenolate ring, as indicated by the results of the corresponding spectroscopic studies. In the presence of iPrOH, 1–4 and 6 could initiate the polymerization of 500 equiv. of rac-lactide to achieve high monomer conversions within several minutes but afford atactic PLAs with slightly isotactic-enriched microstructures (Pm = 0.58–0.60). Experimental results also demonstrated that a bulky trityl substituent at the ortho-position of the phenolate ring of the ligand framework is beneficial for the enhancement of the activities of these potassium complexes.


 Please wait while we load your content...
Please wait while we load your content...