Anion-dependent self-assembly of copper coordination polymers based on pyrazole-3,5-dicarboxylate and 1,2-di(4-pyridyl)ethylene†
Abstract
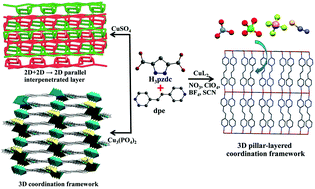
By utilizing a pyrazole-3,5-dicarboxylic acid (H3pzdc) and flexible 1,2-di(4-pyridyl)ethylene (dpe) with various copper(II) salts under the same solvothermal synthetic conditions, six novel coordination polymers, namely, {[Cu2(pzdc)(dpe)2]X}n (X = NO3− (1), ClO4− (2), BF4− (3), SCN− (4)), {[Cu(II)4Cu(I)4(pzdc)4(dpe)6](H2O)4}2n (5), and {[Cu5(HPO4)2 (pzdc)2(dpe)3](H2O)5}n (6) were obtained. The structural diversity of compounds 1–6 depends on the starting Cu(II) salts. Compounds 1–4 are isostructural and exhibit a 3D porous cationic pillar-layered coordination framework with lattice monoanions incorporated into the channels of the framework. When using copper(II) sulfate as a reagent, a neutral mixed-valence Cu(I,II) 2D + 2D → 2D parallel interpenetrated layer of 5 was obtained. In the case of a phosphate trianion, compound 6 shows a 3D coordination framework which contains μ4-HPO42− linking between Cu(II) centers. The anion-exchange properties of 1–4 were studied. Interestingly, compounds 1–4 exhibit the irreversible chemisorption of the thiocyanate anion instead of anion exchange without the destruction of their structural framework as confirmed by PXRD, IR, UV-Vis, and AA spectroscopy. Moreover, the anion-induced structural transformation of 1–4 was observed when exchanging with an azide anion. The luminescent properties of 1–6 and exchanged products were also investigated.


 Please wait while we load your content...
Please wait while we load your content...