Molecular structures of various alkyldichlorosilanes in the solid state†
Abstract
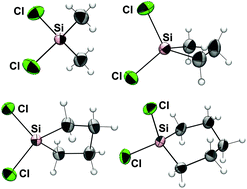
A series of organodichlorosilanes RR′SiCl2 (R,R′ = (CH2)3; (CH2)4; (CH2)5; Me,Me; Me,H; Me,Cl) was studied by single-crystal X-ray diffraction analyses. At ambient temperature liquid chlorosilanes (melting points in the range of 180–220 K) were transferred into glass capillaries and crystallized in situ on a diffractometer. In the solid state structure these chlorosilanes are monomeric, even in the case of the sterically less demanding 1,1-dichlorosilacyclobutane (CH2)3SiCl2. Interestingly, regardless the steric demand of the alkyl substituents, the dialkyldichlorosilanes exhibit essentially the same Cl–Si–Cl angle (for (CH2)3SiCl2, (CH2)4SiCl2, (CH2)5SiCl2, Me2SiCl2: 106.08(3)°, 106.07(4)°/105.86(4)°, 106.91(2)° and 105.59(6)°, respectively). Replacement of one alkyl group by hydrogen has only a marginal influence on the Cl–Si–Cl angle (MeHSiCl2 106.31(3)°), whereas in MeSiCl3 slightly wider Cl–Si–Cl angles are found (ranging between 107.04(11)° and 107.86(11)°), in accordance with VSEPR. Computational analyses, i.e., potential energy surface scans of the Cl–Si–Cl angle variation, of (CH2)3SiCl2, Me2SiCl2, MeHSiCl2 and H2SiCl2 reveal essentially identical energy profiles for the Cl–Si–Cl deformation in these four dichlorosilanes with basically superimposed curves for (CH2)3SiCl2 and Me2SiCl2, whereas with increasing H-substitution the energetic minimum is shifted to a slightly wider Cl–Si–Cl angle. In the crystal packing only MeHSiCl2 exhibits weak intermolecular Si⋯Cl van der Waals contacts, whereas the Si–Cl moieties of the other five chlorosilanes engage in intermolecular Cl⋯H and (for (CH2)3SiCl2, (CH2)4SiCl2 and MeSiCl3) Cl⋯Cl contacts.
- This article is part of the themed collection: Silicon Chemistry: Discoveries and Advances


 Please wait while we load your content...
Please wait while we load your content...