Tuneable reversible redox of cobalt(iii) carbazole complexes†
Abstract
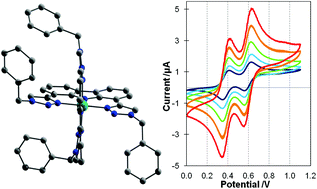
Four tridentate carbazole-based ligands, HLtBu/H {3,6-di(tert-butyl)-1,8-bis[5-(3-benzyl-1,2,3-triazole)]-9H-carbazole}, HLtBu/tBu {3,6-di(tert-butyl)-1,8-bis[5-(3-(4-tert-butyl)benzyl-1,2,3-triazole)]-9H-carbazole}, HLH/H {1,8-bis[5-(3-benzyl-1,2,3-triazole)]-9H-carbazole} and HLH/tBu {1,8-bis[5-(3-(4-tert-butyl)benzyl-1,2,3-triazole)]-9H-carbazole}, were prepared and complexed with cobalt(II) tetrafluoroborate. In situ air oxidation resulted in cobalt(III) complexes 1–4 with the general formula [CoIII(L)2]BF4·xH2O (1: L = LtBu/H, x = 2; 2: L = LtBu/tBu, x = 1; 3: L = LH/H, x = 0.5; 4: L = LH/tBu, x = 2). X-ray structural characterisation confirmed that the four complexes are isostructural, with two orthogonally coordinated deprotonated tridentate ligands providing an octahedral N6-donor set to the cobalt(III) ion. 1H NMR studies show that this structure is maintained in CDCl3 and DMSO-d6 solution. Cyclic voltammetry on 1–4 in MeCN showed that all of the complexes exhibit two reversible, one-electron oxidation processes (probably due to ligand oxidations), and an irreversible or quasi-reversible reduction process (probably due to reduction of Co(III) to Co(II)). As expected, the oxidations move 120–140 mV to lower potentials on adding tert-butyl substituents to the 3 and 6 positions of the carbazole rings, and unsurprisingly the potentials are far less sensitive to the nature of the benzyl ring substituents.


 Please wait while we load your content...
Please wait while we load your content...