Borane-catalyzed indole synthesis through intramolecular hydroamination†
Abstract
The reaction of 2-alkynyl anilines with catalytic amounts of B(C6F5)3 (5 mol%) resulted in the formation of 2-substituted indoles according to an intramolecular hydroamination in good to excellent yields. Reaction intermediates as well as products were characterized by NMR spectroscopy and by X-ray crystallography. The domino hydroamination/hydrogenation sequence allowed the efficient synthesis of tetrahydroquinoline 8 in good yield.
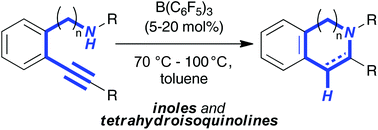


 Please wait while we load your content...
Please wait while we load your content...