Mixing Li and Cs in the reduction of corannulene for the assembly of a cesium-capped sandwich with a hexanuclear heterometallic core†
Abstract
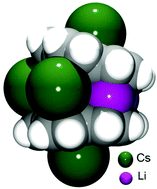
The use of two alkali metals having the greatest ion size mismatch, Li and Cs, in the reduction of bowl-shaped corannulene (C20H10, 1), results in the preparation of a heterobimetallic organometallic self-assembly, [Cs(diglyme)]2//[Li3Cs3(C20H10)2(diglyme)2] (2). The X-ray crystallographic investigation of 2 revealed the formation of a triple-decker sandwich having a highly-charged hexanuclear core, (Li3Cs3)6+, held between two tetrareduced corannulene decks. The sandwich is capped by external cesium ions filling the concave cavities of corannulene bowls. The average depth of two C20H104− anions of 0.754(14) Å is significantly greater in 2 compared to the values of 0.241(2)–0.355(2) Å observed in the homometallic lithium sandwiches formed by tetrareduced corannulene, thus illustrating the unique flexibility of its carbon framework to adapt to different internal and external coordination environments.
- This article is part of the themed collection: Multimetallic complexes: synthesis and applications


 Please wait while we load your content...
Please wait while we load your content...