Unusual N-oxide formation in the peroxidation of cobalt(ii) ethylenediaminetetraacetates†
Abstract
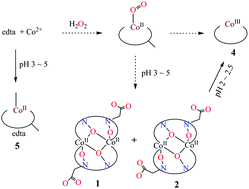
Unlike the usual formations of peroxo and superoxo cobalt products as oxygen carriers, N-oxido cobalt(II) ethylenediaminetetraacetates K4[Co2(edtaO2)2]·nH2O [n = 6.5 (1), 10 (2), H4edta = ethylenediaminetetraacetic acid, C10H16O8N2] and their aggregate [Co2(edtaO2)(H2O)6]n·2nH2O (3) were obtained as dioxygen insertion products. The cobalt ions in dimers 1 and 2 are hexa-coordinated by two edtaO2 ligands in different configurations respectively, which is attributed to the packing of an interesting (H2O)12 water cluster in 2. Further reaction of K4[Co2(edtaO2)2]·nH2O with hydrogen peroxide results in the final trivalent cobalt ethylenediaminetetraacetate K[Co(edta)]·2H2O (4). A water-soluble coordination polymer Na2n[Co(edta)]n·4nH2O (5) was also obtained with a one dimensional zigzag structure. Bond valence calculation is consistent with the products 1–5.

 Please wait while we load your content...
Please wait while we load your content...