Synthesis, reactivity and characterization of Pt(ii) complexes with N,N′ chelating ligands; structure and dimethylsulfoxide reactivity relationship †
Abstract
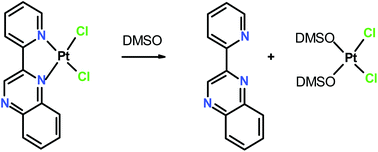
Platinum(II) complexes of the formula PtLCl2 [L = 2-(2′-pyridyl)quinoxaline, (pqx) (1), 2,(2′-pyridyl)benzo[g]quinoxaline, (pbqx) (3) and 2,(2′-pyridyl)quinoline, (pqn) (5)] were synthesized and characterized by spectroscopic and X-ray diffraction methods. Also, monodentate coordination of the ligands pqx and pbqx formed the complexes trans-Pt(DMSO)pqxCl2 (2) and trans-Pt(DMSO)pbqxCl2 (4) as it is indicated from X-ray crystal structure and NMR studies. The reaction of the complexes (1), (3) and (5) with DMSO-d6 revealed a ligand-release solvolysis, which was studied by means of NMR techniques. Correlation between the crystal structures of (1), (3), and (5) and the kinetic or thermodynamic parameters of the solvolysis reactions showed that the tendency of the ligands pqx, pbqx, and pqn to return to the anti-configuration in addition to their ability to form non-classical H-bonds are crucial factors for the ligand-release solvolysis. Instantaneous DMSO-d6 solvolysis for the complexes (1) and (3) and slow kinetics solvolysis for (5) (k = 10−4 ± 6.4 × 10−6 s−1) reflect their structural differences in ligand planarity. Based on NMR techniques a two-step mechanism of the chelate ring opening was suggested with equilibrium constants of the overall reaction at 298 K, Keq = 4.1 ± 0.2 × 10−4 M−1 (1) and Keq = 1.7 ± 0.2 × 10−4 M−1 (2).


 Please wait while we load your content...
Please wait while we load your content...