Insight into the structure and molybdenum species in mesoporous molybdena–alumina catalysts for isobutane dehydrogenation†
Abstract
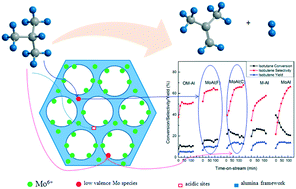
The relationship between the structure and Mo species in mesoporous molybdena–alumina catalysts and their catalytic performance for isobutane dehydrogenation has been investigated in detail. Characterization by XRD, HAADF-STEM, EDX, FT-IR, and N2 physisorption illustrated that ordered mesoporous catalysts (OM-Al, MoAl(F), and MoAl(C)) possessed an amorphous alumina phase and non-ordered mesoporous catalysts (M-Al and MoAl) exhibited a γ-Al2O3 phase. Mo species were highly dispersed over all the catalysts because Mo surface densities were about 1.0 Mo nm−2. Moreover, XPS and ICP-OES showed that Mo species were uniformly distributed over MoAl(F) with the Mo species confined in the ordered mesoporous structure. Higher dehydrogenation stability and a lower coke formation rate, albeit lower catalytic conversion, was obtained over MoAl(F) in comparison with those of MoAl(C) and MoAl on account of its stronger metal–support interaction, as shown by H2-TPR technique. The catalyst with a γ-Al2O3 phase exhibited stronger acidity and higher activity than the corresponding catalyst with an amorphous phase. The acidity of the catalysts was greatly enhanced by the addition of Mo species, according to the NH3-TPD characterization. However, not all the acid sites were active sites for dehydrogenation activity. The moderately and strongly acidic sites and the Mo species, including Mo6+ and lower valence Mo species, probably contributed to the dehydrogenation reactivity. Moreover, deactivation of the catalysts was mainly due to coke formation over the spent catalysts.


 Please wait while we load your content...
Please wait while we load your content...