Ambient temperature NO oxidation over Cr-based amorphous mixed oxide catalysts: effects from the second oxide components
Abstract
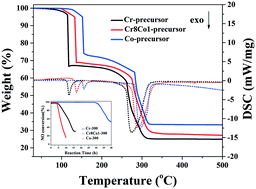
Three series of Cr-based mixed oxides (Cr–Co, Cr–Fe, and Cr–Ni oxides) with high specific surface areas and amorphous textures were synthesized using a novel sol–gel method. These mixed oxides, in comparison to their pure metal oxide (CrOx, Co3O4, FeOx and NiO) counterparts, display enhanced performance for catalytic oxidation of low-concentration NO at room temperature. The best performing catalysts achieve 100% NO conversion for ∼30 h of operation at a high space velocity of 45 000 ml g−1 h−1. The amorphous structure was found to be critical for these catalysts to maintain high activity and durability. Control of the Cr/M (M = Co, Fe and Ni) molar ratio, nitrate precursor decomposition temperature and catalyst calcination temperature was key to the synthesis of these highly active catalysts.


 Please wait while we load your content...
Please wait while we load your content...