Aldose to ketose interconversion: galactose and arabinose isomerization over heterogeneous catalysts
Abstract
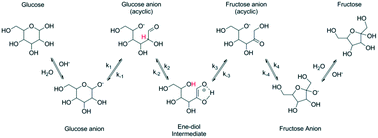
Isomerization of glucose, galactose and arabinose to corresponding keto-sugars was studied in the present work over a range of heterogeneous catalysts. Magnesium aluminates with different ratios between oxides resulting in materials with a Mg/Al ratio from 0.2 to 0.9 were prepared, characterized and evaluated in terms of their catalytic behavior. The catalyst with a Mg/Al ratio close to hydrotalcites was the most efficient considering activity, selectivity and stability. The sugar structure was shown to have a minor influence on catalytic activity and selectivity.
- This article is part of the themed collection: Catalytic reactivity of surfaces: in recognition of François Gault


 Please wait while we load your content...
Please wait while we load your content...