Catalytic conversion of methanol to aromatics over nano-sized HZSM-5 zeolite modified by ZnSiF6·6H2O
Abstract
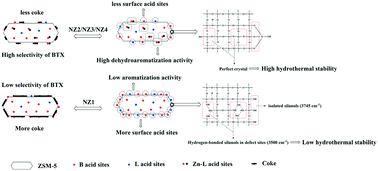
ZnSiF6-modified nano-sized HZSM-5 zeolites (NZ2, NZ3 and NZ4 catalysts) were prepared and investigated as catalysts for the conversion of methanol to aromatics. Moreover, the ZnNZ1 catalyst, prepared by ion exchange using zinc nitrate, was also introduced as a reference sample. The effects of modification on the framework, textural properties and acidity of the parent nano-sized HZSM-5 zeolite (NZ1) were investigated by XRD, FT-IR, 29Si MAS-NMR, SEM, N2 adsorption–desorption, ICP, NH3-TPD, infrared spectroscopy of adsorbed pyridine (Py-IR), UV-vis spectra, X-ray photoelectron spectroscopy (XPS) and n-butylamine and tert-butylamine titration. The results showed that the amount of total acid sites, especially the external surface acid sites of the NZ2, NZ3 and NZ4 catalysts, significantly decreased, which may largely be attributed to the passivation effect of SiF62− on the surface acidity of the parent NZ1 catalyst. Moreover, the amount of Lewis acid sites (L acid sites) increased, whereas the amount of Brønsted acid sites (B acid sites) obviously decreased with the introduction of zinc species. The emergence of new Zn-Lewis acid sites (⊖ZO⋯H⋯O–Zn⊕ species) was beneficial to improving the selectivity to BTX (benzene (B), toluene (T) and xylene (X)) due to their high activity for dehydroaromatization. The FT-IR spectra in the OH− vibration region and the 29Si MAS-NMR spectra show that the treatment of ZnSiF6 could effectively repair partial lattice defects of zeolite and could thus improve the catalyst stability. TG analysis of all the deactivated catalysts showed that the coke amount and the average rate of coke formation decreased over NZ2, NZ3 and NZ4 catalysts, and this may largely be ascribed to their lower surface acidity. The catalytic performance of these materials on the conversion of methanol to aromatics showed that the NZ3 catalyst had the highest selectivity to BTX of about 51.3% and the longest catalytic lifetime of about 234 h under the operating conditions of T = 425 °C, p = 0.1 MPa and WHSV = 0.8 h−1. The improvement in the selectivity to BTX and the catalyst lifetime of the NZ3 catalyst could be ascribed to the synergistic effect among the Zn-Lewis acid sites (⊖ZO⋯H⋯O–Zn⊕ species), external surface acidity and intact framework structure.


 Please wait while we load your content...
Please wait while we load your content...