Hierarchical yolk–shell layered potassium niobate for tuned pH-dependent photocatalytic H2 evolution†
Abstract
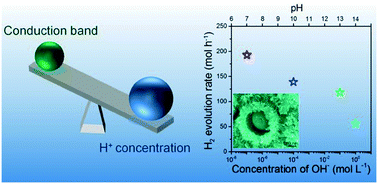
Photocatalysts and the reaction environments in which they act are crucial for improving the photocatalytic efficiency. But the pH-dependent evolution of H2 photocatalysed using nanoscale potassium niobate particles with high surface areas has not received attention. In this study, a straightforward Ostwald ripening method was developed to synthesize KNb3O8 with a thin nanosheet assembled hierarchical yolk–shell structure and large surface area of 60.6 m2 g−1. The H2 evolution from a water–methanol solution in an alkaline to neutral environment was studied. The photocatalytic H2 evolution rates over fabricated hierarchical yolk–shell KNb3O8 increased when OH− concentrations were decreased. Such behaviour implied that the concentration of H+ dominated the H2 evolution over hierarchical yolk–shell KNb3O8 rather than the reduction ability from the conduction band, differing from the corresponding bulk material. This study demonstrated an efficient method to achieve a high H2 evolution rate in a neutral environment through the use of photocatalysts with hierarchical structures and large surface areas.


 Please wait while we load your content...
Please wait while we load your content...