Aluminium fluoride – the strongest solid Lewis acid: structure and reactivity
Abstract
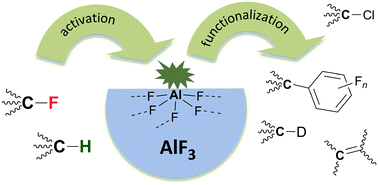
High surface aluminium fluoride, HS-AlF3, and aluminium chlorofluoride, ACF, (AlClxF3−x, x = 0.05…0.3) are amorphous solids with an extraordinary high Lewis acidity, which play an increasingly important role in heterogeneous catalysis. After a historic introduction, an overview of the different experimental and theoretical methods for understanding the nature of these catalysts is provided. Special attention is given to the theoretical modelling of the surface centres and the comparison with experimental results. A classification of these solid Lewis acids among well-known molecular Lewis acids is achieved using various methods. Several fascinating catalytic reactions involving C–H and C–F bond activation are presented in the second part of the review. This includes H/D exchange between alkanes (and not only arenes), hydroarylation even with deactivated aromatic compounds, activation of fluoromethanes due to hydrodefluorination, and finally, selective dehydrohalogenation reactions of hydrochlorofluoro-alkanes.
- This article is part of the themed collection: 2017 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...