Towards stoichiometric analogues of graphene: graphane, fluorographene, graphol, graphene acid and others
Abstract
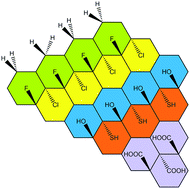
Stoichiometric derivatives of graphene, having well-defined chemical structure and well-defined chemical bonds, are of a great interest to the 2D materials research. Hydrogenated graphene (graphene), fluorographene, hydroxygraphene (graphol), thiographene, cyanographene, aminographene, graphene acid pose unique and well defined chemical and physical properties, allowing their further transformation and application in the devices. Here we overview various methods of their synthesis and discuss their chemistry and properties. It is expected that the family of stochiometric derivatives of graphene will grow beyond listed examples in the near future.
- This article is part of the themed collection: Graphene Chemistry


 Please wait while we load your content...
Please wait while we load your content...