α-Substituted vinyl azides: an emerging functionalized alkene
Abstract
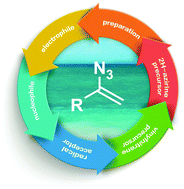
Vinyl azides are highly versatile synthons that provide access to numerous N-heterocycles and other functional groups. α-Substituted vinyl azides (azido vinylidenes) are a special class that display unique reactivity, able to react not only as azides, but also as radical acceptors, enamine-type nucleophiles, and even electrophiles, thus delivering a wide range of nitrogen-containing compounds and their derivatives. An impressive variety of intermediates – such as iminodiazonium ions, nitrilium ions, iminyl radicals, and metal enaminyl radicals – can be generated from vinyl azides and exploited in cycloadditions, C–H functionalizations, hydrolysis processes, and cascade reactions under transition metal/photoredox catalysis. In addition to presenting synthetic protocols to access vinyl azides, this Review offers a comprehensive coverage of the development of their multifaceted reactivity, and highlights their potential as versatile precursors for synthetic applications.


 Please wait while we load your content...
Please wait while we load your content...