A high-performance electrochemical supercapacitor based on a polyaniline/reduced graphene oxide electrode and a copper(ii) ion active electrolyte
Abstract
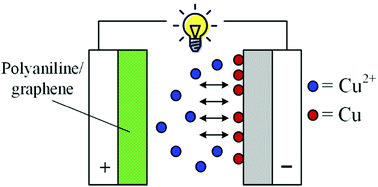
Active electrolyte enhanced supercapacitors (AEESCs) have received increasing attention because of their large specific capacitance and easy fabrication process. The better matching between the active electrolyte and the counter electrode and the slow self-discharge rate are the challenges of this type of supercapacitor. In this paper, a novel AEESC with polyaniline/reduced graphene oxide hydrogel (PANI/RGOHG) as the anode and Cu(II) ions as the cathodic active electrolyte is constructed. Experimental results demonstrate that the electrode potentials of PANI and Cu(II) can match perfectly, thus the device has a wide working voltage range. Because of the large specific capacitance of both PANI and Cu(II), a high average specific capacitance of a single electrode of 1120 F g−1 at 2.6 A g−1 is achieved. Meanwhile, self-discharge is also suppressed because the reduction product of Cu(II) is immobilized on the electrode. These results demonstrate that the performance of AEESCs strongly depends on the choice of a suitable electrode material, and also reveal that Cu(II) is a promising cathodic active electrolyte for AEESCs.


 Please wait while we load your content...
Please wait while we load your content...