From ligand exchange to reaction intermediates: what does really happen during the synthesis of emissive complexes?†‡
Abstract
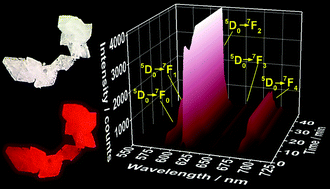
In situ monitoring of the formation of emissive complexes is essential to enable the development of rational synthesis protocols, to provide accurate control over the generation of structure-related properties (such as luminescence) and to facilitate the development of new compounds. In situ luminescence analysis of coordination sensors (ILACS) utilizes the sensitivity of the spectroscopic properties of lanthanide ions to their coordination environment to detect structural changes during crystallization processes. Here, ILACS was utilized to monitor the formation of [Eu(bipy)2(NO3)3] (bipy = 2,2′-bipyridine) during co-precipitation synthesis. Validity of the ILACS results was ensured by concomitant utilization of in situ monitoring of other reaction parameters, including in situ measurements of pH value, ionic conductivity, and infrared spectra, as well as ex situ and synchrotron-based in situ X-ray diffraction analyses. Gradual desolvation of the Eu3+ ions and attachment of ligands were detected by an exponential increase of the intensity of the 5D0 → 7FJ (J = 0–4) transitions in the emission spectrum. Additionally, the in situ emission spectra show a decrease in the crystallization rate and an increase in the induction time in response to a reduction in the concentration of the starting solutions from 12 mM until crystallization ceased at starting reactant concentrations <6 mM. An increase to a three-fold higher concentration leads to the formation of a reaction intermediate, and its stability was determined to be highly concentration-dependent. The in situ luminescence measurements also demonstrated the existence of a ligand exchange process within the [Eu(bipy)2(NO3)3] complex upon addition of a phen (phen = 1,10′-phenanthroline) solution and the generation of a new phen-containing emissive complex. In attempting to solve the structure of this new phen-containing complex, a different, but nevertheless previously unsynthesized complex, [Eu(phen)2(NO3)3]bipy, was obtained, which shows characteristic Eu3+ luminescence in the red spectral range.


 Please wait while we load your content...
Please wait while we load your content...