HyRes: a coarse-grained model for multi-scale enhanced sampling of disordered protein conformations†
Abstract
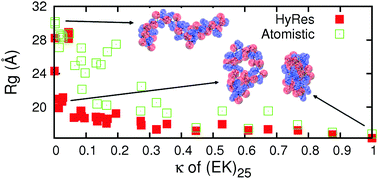
Efficient coarse-grained (CG) models can be coupled with atomistic force fields to accelerate the sampling of atomistic energy landscapes in the multi-scale enhanced sampling (MSES) framework. This approach may be particularly suitable for generating atomistic conformational ensembles of intrinsically disordered proteins (IDPs). While MSES is relatively robust to inherent CG artifacts, achieving optimal sampling efficiency requires CG modeling to generate the local and long-range fluctuations that are largely consistent with those at the atomistic level. Here, we describe a new hybrid resolution CG model (HyRes) for MSES simulations of disordered protein states, which is specifically designed to provide semi-quantitative secondary structure propensities together with a qualitative description of long-range nonspecific interactions. The HyRes model contains an atomistic description of the backbone with intermediate resolution side chains. The secondary structure propensities are tuned by adjusting the backbone hydrogen-bonding strength and the ϕ/ψ torsion profile. The sizes and covalent geometries of the side chains are parameterized to reproduce distributions derived from atomistic simulations. Lennard-Jones parameters for sidechain beads are assigned to reproduce statistical potentials derived from the protein structural database, and then globally parameterized with nonspecific electrostatic interactions to reproduce the free energy profiles of pair wise interactions and the key conformational properties of model peptides. Application of HyRes to MSES simulations of small IDPs suggests that it is capable of driving faster structural transitions at the atomistic level and increasing the convergence rate compared to the Cα-only Gō-like models previously utilized. With further optimization, we believe that the new CG model could greatly improve the efficiency of MSES simulations of the larger and more complex IDPs frequently involved in cellular signalling and regulation.


 Please wait while we load your content...
Please wait while we load your content...