Cooperating dipole–dipole and van der Waals interactions driven 2D self-assembly of fluorenone derivatives: ester chain length effect†
Abstract
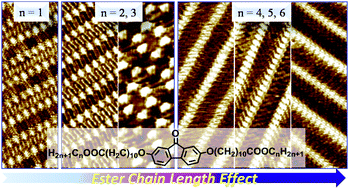
Two-dimensional supramolecular assemblies of a series of 2,7-bis(10-n-alkoxycarbonyl-decyloxy)-9-fluorenone derivatives (BAF-Cn, n = 1, 3–6) consisting of polar fluorenone moieties and ester alkoxy chains were investigated by scanning tunneling microscopy on highly oriented pyrolytic graphite surfaces. The chain-length effect was observed in the self-assembly of BAF-Cn. Self-assembly of BAF-C1 was composed of a linear I pattern, where the side chains adopted a fully interdigitated arrangement. As the length of side chains increased, the coexistence of a linear I pattern and a cyclic pattern for the self-assembly of BAF-C3 was observed. Upon increasing the length of the alkoxy chain even further (n = 4–6), another linear II structure was observed in the BAF-Cn monolayer, in which the side chains in adjacent rows were arranged in a tail-to-tail configuration. It is reasonable to conclude that not only the van der Waals forces but also the dipole–dipole interactions from both the fluorenone cores and the ester alkoxy chains play critical roles in the self-assemblies of BAF-Cn. Our work provides detailed insights into the effect of intermolecular dipole–dipole and van der Waals interactions on the monolayer morphology of fluorenone derivatives.


 Please wait while we load your content...
Please wait while we load your content...